Description
Cyanocobalamin is a synthetic form of vitamin B12 with equivalent vitamin
B12 activity. The
chemical name is 5,6-dimethyl-benzimidazolyl cyanocobamide. The cobalt content is 4.35%.
The
molecular formula is C63H88CoN14O14P, which
corresponds to a molecular weight of 1355.38 and the
following structural formula:
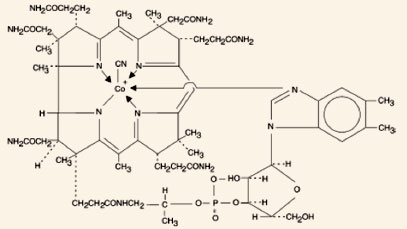
Cyanocobalamin occurs as dark red crystals or orthorhombic needles or crystalline red
powder. It
is very hygroscopic in the anhydrous form, and sparingly to moderately soluble in water
(1:80).
Its pharmacologic activity is destroyed by heavy metals (iron) and strong oxidizing or
reducing
agents (vitamin C), but not by autoclaving for short periods of time (15-20 minutes) at
121°C.
The vitamin B12 coenzymes are very unstable in light.
Nascobal® Nasal Spray is a solution of Cyanocobalamin, USP (vitamin B12)
for administration as a
spray to the nasal mucosa. Each bottle of Nascobal Nasal Spray contains 1.3 mL of a 500
mcg/0.1
mL solution of cyanocobalamin with sodium citrate, citric acid, glycerin and
benzalkonium
chloride in purified water. The spray solution has a pH between 4.5 and 5.5. The spray
pump unit
must be fully primed (see Dosage and
Administration) prior to initial
use. After initial
priming, each spray delivers an average of 500 mcg of cyanocobalamin and the 1.3 mL of
spray
solution contained in the bottle will deliver 4 doses of Nascobal Nasal Spray. The unit
must be
re-primed before each dose. (see Dosage and
Administration).
CLINICAL PHARMACOLOGY
GENERAL PHARMACOLOGY AND MECHANISM OF ACTION
Vitamin B12 is essential to growth, cell reproduction, hematopoiesis, and
nucleoprotein and
myelin synthesis. Cells characterized by rapid division
(e.g., epithelial cells, bone marrow, myeloid cells) appear to have the greatest
requirement for
vitamin B12. Vitamin B12 can be converted to coenzyme
B12 in
tissues, and as such is essential
for conversion of methylmalonate to succinate and synthesis of methionine from
homocysteine, a
reaction which also requires folate. In the absence of coenzyme B12,
tetrahydrofolate
cannot be
regenerated from its inactive storage form, 5-methyl tetrahydrofolate, and a functional
folate
deficiency occurs. Vitamin B12 also may be involved in maintaining sulfhydryl
(SH)
groups in the
reduced form required by many SH-activated enzyme systems. Through these reactions,
vitamin
B12
is associated with fat and carbohydrate metabolism and protein synthesis. Vitamin B12
deficiency
results in megaloblastic anemia, GI lesions, and neurologic damage that begins with an
inability
to produce myelin and is followed by gradual degeneration of the axon and nerve head.
Cyanocobalamin is the most stable and widely used form of vitamin B12, and has
hematopoietic activity apparently identical to that of the
antianemia factor in purified liver extract. The information below, describing the
clinical
pharmacology of cyanocobalamin, has been derived from studies with injectable vitamin
B12.
Vitamin B12 is quantitatively and rapidly absorbed from intramuscular and
subcutaneous
sites of
injection. It is bound to plasma proteins and stored in the liver. Vitamin
B12 is
excreted in
the bile and undergoes some enterohepatic recycling. Absorbed vitamin B12 is
transported via
specific B12 binding proteins, transcobalamin I and II, to the various
tissues. The
liver is the
main organ for vitamin B12 storage.
Parenteral (intramuscular) administration of vitamin B12 completely reverses
the
megaloblastic
anemia and GI symptoms of vitamin B12 deficiency; the degree of improvement
in
neurologic
symptoms depends on the duration and severity of the lesions, although progression of
the
lesions is immediately arrested.
Gastrointestinal absorption of vitamin B12 depends on the presence of
sufficient
intrinsic factor
and calcium ions. Intrinsic factor deficiency causes
pernicious anemia, which may be associated with subacute combined degeneration of the
spinal
cord. Prompt parenteral administration of vitamin
B12 prevents progression of neurologic damage.
The average diet supplies about 4 to 15 mcg/day of vitamin B12 in a
protein-bound form
that is
available for absorption after normal digestion.
Vitamin B12 is not present in foods of plant origin, but is abundant in foods
of
animal origin.
In people with normal absorption, deficiencies have been reported only in strict
vegetarians who
consume no products of animal origin (including no milk products or eggs).
Vitamin B12 is bound to intrinsic factor during transit through the stomach;
separation occurs in
the terminal ileum in the presence of calcium, and vitamin B12 enters the
mucosal
cell for
absorption. It is then transported by the transcobalamin binding proteins. A small
amount
(approximately
1% of the total amount ingested) is absorbed by simple diffusion, but this mechanism is
adequate
only with very large doses. Oral absorption is considered too undependable to rely on in
patients with pernicious anemia or other conditions resulting in malabsorption of
vitamin
B12 .
Colchicine, para-aminosalicylic acid, and heavy alcohol intake for longer than 2 weeks
may
produce malabsorption of vitamin B12 .
Pharmacokinetics
Absorption
A three way crossover study in 25 fasting healthy subjects was conducted to compare the
bioavailability of the B12 nasal spray to the B12 nasal gel and to
evaluate the relative
bioavailability of the nasal formulations as compared to the intramuscular injection.
The peak
concentrations after administration of intranasal spray were reached in 1.25 +/- 1.9
hours. The
average peak concentration of B12 obtained after baseline correction
following
administration of
intranasal spray was 757.96 +/- 532.17 pg/mL. The bioavailability of the nasal spray
relative to
the intramuscular injection was found to be 6.1%. The bioavailability of the
B12
nasal spray was
found to be 10% less than the B12 nasal gel. The 90% confidence intervals for
the
loge -
transformed AUC(0-t) and Cmax was 71.71% - 114.19% and 71.6% - 118.66% respectively.
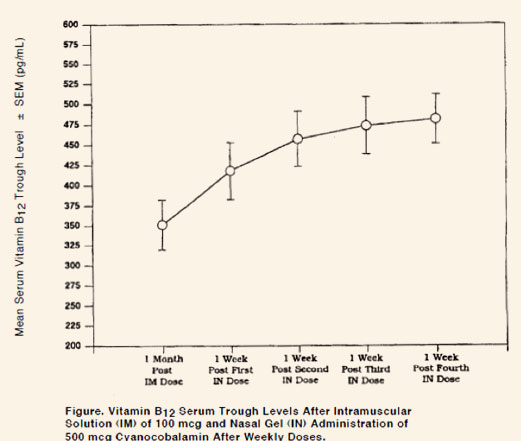
In pernicious anemia patients, once weekly intranasal dosing with 500 mcg B12
gel
resulted in a
consistent increase in pre-dose serum B12 levels during one month of
treatment (p < 0.003) above
that seen one month after 100 mcg intramuscular dose (Figure).
Distribution
In the blood, B12 is bound to transcobalamin II, a specific B-globulin carrier
protein, and is
distributed and stored primarily in the liver and bone
marrow.
Elimination
About 3-8 mcg of B12 is secreted into the GI tract daily via the bile; in
normal
subjects with
sufficient intrinsic factor, all but about 1 mcg is reabsorbed
When B12 is administered in doses which saturate the binding capacity of
plasma
proteins and the
liver, the unbound B12 is rapidly eliminated in the urine. Retention of
B12 in the body is
dose-dependent. About 80-90% of an intramuscular dose up to 50 mcg is retained in the
body; this
percentage drops to 55% for a 100 mcg dose, and decreases to 15% when a 1000 mcg dose is
given.
INDICATIONS AND USAGE
Nascobal Nasal Spray is indicated for the maintenance of normal hematologic status in
pernicious
anemia patients who are in remission following intramuscular vitamin B12
therapy and who have no
nervous system involvement.
Nascobal Nasal Spray is also indicated as a supplement for other vitamin B12
deficiencies,
including:
- Dietary deficiency of vitamin B12 occurring in strict vegetarians
(Isolated vitamin
B12
deficiency is very rare).
- Malabsorption of vitamin B12 resulting from structural or functional
damage to the stomach,
where intrinsic factor is secreted, or to the ileum, where intrinsic factor
facilitates
vitamin B12 absorption. These conditions include HIV infection, AIDS,
Crohn’s disease,
tropical
sprue, and nontropical sprue (idiopathic steatorrhea, gluten-induced enteropathy).
Folate
deficiency in these patients is usually more severe than vitamin B12
deficiency.
- Inadequate secretion of intrinsic factor, resulting from lesions that destroy the
gastric mucosa (ingestion
of
corrosives, extensive neoplasia), and a number of conditions associated with a
variable degree of gastric
atrophy (such as multiple sclerosis, HIV infection, AIDS, certain endocrine
disorders, iron deficiency, and
subtotal gastrectomy). Total gastrectomy always produces vitamin B12
deficiency. Structural
lesions leading to
vitamin B12 deficiency include regional ileitis, ileal resections,
malignancies, etc.
- Competition for vitamin B12 by intestinal parasites or bacteria. The fish
tapeworm
(Diphyllobothrium latum)
absorbs huge quantities of vitamin B12 and infested patients often have
associated gastric
atrophy. The blind
loop syndrome may produce deficiency of vitamin B12 or folate.
- Inadequate utilization of vitamin B12. This may occur if antimetabolites
for the vitamin are
employed in the
treatment of neoplasia.
It may be possible to treat the underlying disease by surgical correction of anatomic
lesions leading to
small bowel
bacterial overgrowth, expulsion of fish tapeworm, discontinuation of drugs leading to
vitamin malabsorption
(see
"Drug/Laboratory Test Interactions"), use of a gluten free diet in
nontropical sprue, or administration of antibiotics in tropical sprue. Such measures
remove the need for
long-term
administration of vitamin B12.
Requirements of vitamin B12 in excess of normal (due to pregnancy,
thyrotoxicosis, hemolytic
anemia, hemorrhage,
malignancy, hepatic and renal disease) can usually be met with intranasal or oral
supplementation.
Nascobal Nasal Spray is not suitable for vitamin B12 absorption test
(Schilling Test).
Contraindication
Sensitivity to cobalt and/or vitamin B12 or any component of the medication is
a contraindication.
WARNINGS
Patients with early Leber’s disease (hereditary optic nerve atrophy) who were treated
with vitamin B12
suffered severe and swift optic atrophy.
Hypokalemia and sudden death may occur in severe megaloblastic anemia which is treated
intensely with
vitamin B12. Folic acid is not a
substitute for vitamin B12 although it may improve vitamin B12-deficient
megaloblastic
anemia. Exclusive use
of folic acid in treating vitamin B12-
deficient megaloblastic anemia could result in progressive and irreversible neurologic
damage.
Anaphylactic shock and death have been reported after parenteral vitamin B12
administration. No such
reactions have been reported in clinical
trials with Nascobal Nasal Spray or Nascobal Nasal Gel.
Blunted or impeded therapeutic response to vitamin B12 may be due to such
conditions as infection,
uremia,
drugs having bone marrow
suppressant properties such as chloramphenicol, and concurrent iron or folic acid
deficiency.
Precautions
1. GENERAL
An intradermal test dose of parenteral vitamin B12 is recommended before
Nascobal Nasal Spray is
administered
to patients suspected of
cyanocobalamin sensitivity. Vitamin B12 deficiency that is allowed to
progress for longer than
three months
may produce permanent degenerative
lesions of the spinal cord.
Doses of folic acid greater than 0.1 mg per day may result in hematologic remission in
patients with vitamin
B12
deficiency. Neurologic manifestations will not be prevented with folic acid, and if not
treated with vitamin
B12, irreversible damage will result.
Doses of vitamin B12 exceeding 10 mcg daily may produce hematologic response
in patients with
folate
deficiency. Indiscriminate administration
may mask the true diagnosis.
The validity of diagnostic vitamin B12 or folic acid blood assays could be
compromised by
medications, and
this should be considered before
relying on such tests for therapy.
Vitamin B12 is not a substitute for folic acid and since it might improve
folic acid deficient
megaloblastic
anemia, indiscriminate use of vitamin B12
could mask the true diagnosis.
Hypokalemia and thrombocytosis could occur upon conversion of severe megaloblastic to
normal erythropoiesis
with vitamin B12 therapy.
Therefore, serum potassium levels and the platelet count should be monitored carefully
during therapy.
Vitamin B12 deficiency may suppress the signs of polycythemia vera. Treatment
with vitamin
B12
may unmask
this condition.
If a patient is not properly maintained with Nascobal® Nasal Spray,
intramuscular vitamin
B12 is necessary
for adequate treatment of the patient.
No single regimen fits all cases, and the status of the patient observed in follow-up is
the final criterion
for adequacy of therapy.
The effectiveness of Nascobal Nasal Spray in patients with nasal congestion, allergic
rhinitis and upper
respiratory infections has not been determined. Therefore, treatment with Nascobal Nasal
Spray should be
deferred until symptoms have subsided.
2. INFORMATION FOR PATIENTS
Patients with pernicious anemia should be instructed that they will require weekly
intranasal administration
of Nascobal Nasal Spray for the remainder of their lives. Failure to do so will result
in return of the
anemia and in development of incapacitating and irreversible damage to the nerves of the
spinal cord. Also,
patients should be warned about the danger of taking folic acid in place of vitamin
B12, because the
former
may prevent anemia but allow progression of subacute combined degeneration of the spinal
cord.
(Hot foods may cause nasal secretions and a resulting loss of medication; therefore,
patients should be told
to administer Nascobal Nasal Spray at least one hour before or one hour after ingestion
of hot foods or
liquids).
A vegetarian diet which contains no animal products (including milk products or eggs)
does not supply any
vitamin B12. Therefore, patients following such a diet should be advised to
take Nascobal Nasal Spray
weekly. The need for vitamin B12 is increased by pregnancy and lactation.
Deficiency has been recognized in infants of vegetarian mothers who were breast fed,
even though the mothers
had no symptoms of deficiency at the time.
Because the nasal dosage forms of Vitamin B12 have a lower absorption than
intramuscular dosage, nasal
dosage
forms are administered weekly, rather than the monthly intramuscular dosage. As shown in
the Figure above,
at the end of a month, weekly nasal administration results in significantly higher serum
Vitamin B12
levels
than after intramuscular administration. The patient should also understand the
importance of returning for
follow-up blood tests every 3 to 6 months to confirm adequacy of the therapy.
Careful instructions on the actuator assembly, removal of the safety clip, priming of the
actuator and nasal
administration of Nascobal Nasal Spray should be given to the patient. Although
instructions for patients
are supplied with individual bottles, procedures for use should be demonstrated to each
patient.
3. LABORATORY TESTS
Hematocrit, reticulocyte count, vitamin B12, folate and iron levels should be
obtained prior to
treatment. If
folate levels are low, folic acid should also be administered. All hematologic
parameters should be normal
when beginning treatment with Nascobal® Nasal Spray.
Vitamin B12 blood levels and peripheral blood counts must be monitored
initially at one month after
the start
of treatment with Nascobal® Nasal Spray, and then at intervals of 3 to 6 months.
A decline in the serum levels of B12 after one month of treatment with B12
nasal spray may
indicate that the
dose may need to be adjusted upward. Patients should be seen one month after each dose
adjustment; continued
low levels of serum B12 may indicate that the patient is not a candidate for
this mode of
administration.
Patients with pernicious anemia have about 3 times the incidence of carcinoma of the
stomach as in the
general population, so appropriate tests for this condition should be carried out when
indicated.
4. DRUG/LABORATORY TEST INTERACTIONS
Persons taking most antibiotics, methotrexate or pyrimethamine invalidate folic acid and
vitamin B12
diagnostic blood assays.
Colchicine, para-aminosalicylic acid and heavy alcohol intake for longer than 2 weeks may
produce
malabsorption of vitamin B12.
5. CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
Long-term studies in animals to evaluate carcinogenic potential have not been done. There
is no evidence from
long-term use in patients with pernicious anemia that vitamin B12 is
carcinogenic. Pernicious anemia
is
associated with an increased incidence of carcinoma of the stomach, but this is believed
to be related to
the underlying pathology and not to treatment with vitamin B12.
6. PREGNANCY
Pregnancy Category C: Animal reproduction studies have not been conducted with vitamin
B12. It is also
not
known whether vitamin B12 can cause fetal harm when administered to a
pregnant woman or can affect
reproduction capacity. Adequate and well-controlled studies have not been done in
pregnant women. However,
vitamin B12 is an essential vitamin and requirements are increased during
pregnancy. Amounts of
vitamin B12
that are recommended by the Food and Nutrition Board, National Academy of Science -
National Research
Council for pregnant women should be consumed during pregnancy.
7. NURSING MOTHERS
Vitamin B12 appears in the milk of nursing mothers in concentrations which
approximate the mother’s
vitamin
B12 blood level. Amounts of vitamin
B12 that are recommended by the Food and Nutrition Board, National Academy of
Science-National
Research
Council for lactating women should be consumed during lactation.
8. PEDIATRIC USE
Intake in pediatric patients should be in the amount recommended by the Food and
Nutrition Board, National
Academy of Science-National Research Council.
Adverse Reactions
The incidence of adverse experiences described in the Table below are based on data from
a short-term
clinical trial in vitamin B12 deficient patients in hematologic remission
receiving Nascobal
(Cyanocobalamin, USP) Gel for Intranasal Administration (N=24) and intramuscular vitamin
B12 (N=25). In the pharmacokinetic study comparing Nascobal Nasal Spray and
Nascobal Nasal Gel, the
incidence of adverse events was similar.
Table: Adverse Experiences by Body System, Number of Patients, and Number of
Occurrences by Treatment
Following Intramuscular and Intranasal Administration of Cyanocobalamin.
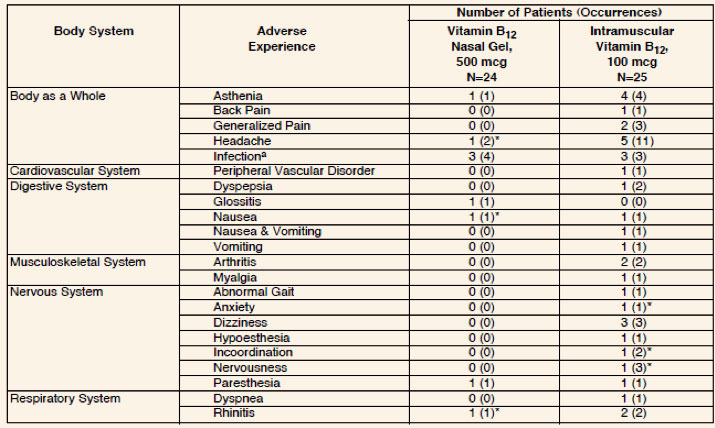
a Sore throat, common cold
* There may be a possible relationship between these adverse experiences and
the study drugs.
These adverse experiences could have also been produced by the patient’s clinical state
or other concomitant
therapy.
The intensity of the reported adverse experiences following the administration of
Nascobal (Cyanocobalamin,
USP) Gel for Intranasal
Administration and intramuscular vitamin B12 were generally mild. One patient
reported severe
headache
following intramuscular dosing. Similarly, a few adverse experiences of moderate
intensity were reported
following intramuscular dosing (two headaches and rhinitis; one dyspepsia, arthritis,
and dizziness), and
dosing with Nascobal (Cyanocobalamin, USP) Gel for Intranasal Administration (one
headache, infection, and
paresthesia).
The majority of the reported adverse experiences following dosing with Nascobal
(Cyanocobalamin, USP) Gel for
Intranasal Administration and intramuscular vitamin B12 were judged to be
intercurrent events. For
the other
reported adverse experiences, the relationship to study drug was judged as "possible"
or "remote".
Of the
adverse experiences judged to be of "possible" relationship to the study drug,
anxiety,
incoordination, and
nervousness were reported following intramuscular vitamin B12 and headache,
nausea, and rhinitis were
reported following dosing with Nascobal
(Cyanocobalamin, USP) Gel for Intranasal Administration.
The following adverse reactions have been reported with parenteral vitamin B12:
| Generalized: |
Anaphylactic shock and death (See Warnings and Precautions). |
| Cardiovascular: |
Pulmonary edema and congestive heart failure early in treatment; peripheral
vascular thrombosis.
|
| Hematological: |
Polycythemia vera. |
| Gastrointestinal: |
Mild transient diarrhea. |
| Dermatological: |
Itching; transitory exanthema. |
| Miscellaneous: |
Feeling of swelling of the entire body. |
Overdosage
No overdosage has been reported with Nascobal Nasal Spray, Nascobal (Cyanocobalamin, USP)
Gel for Intranasal
Administration or parenteral
vitamin B12.
Dosage and Administration
The recommended initial dose of Nascobal Nasal Spray is one spray (500 mcg) administered
in ONE nostril once
weekly. Nascobal Nasal Spray should be administered at least one hour before or one hour
after ingestion of
hot foods or liquids. Periodic monitoring of serum B12 levels should be
obtained to establish
adequacy of
therapy.
Priming (Activation) of Pump
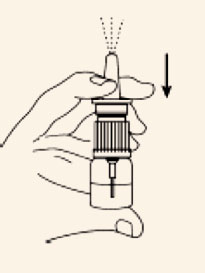
Before the first dose and administration, the pump must be primed.
Remove the clear plastic cover and the plastic safety clip from the pump. To prime the
pump,
place nozzle between the first and second finger with the thumb on the bottom of the
bottle.
Pump the unit firmly and quickly until the first appearance of spray. Then prime the
pump an additional 2
times. Now the nasal spray is ready for use. The unit must be re-primed before each
dose. Prime the pump
once immediately before each administration of doses 2 through 4.
See LABORATORY TESTS for monitoring B12
levels and adjustment of
dosage.
How Supplied
Nascobal Nasal Spray is available as a spray in 3 mL glass bottles containing 1.3 mL of
solution. It is available
in a dosage strength of 500 mcg per actuation (0.1 mL/actuation). A screw-on actuator is
provided. This
actuator, following priming, will deliver 0.1 mL of the spray. Nascobal Nasal Spray is
provided in a carton
containing a nasal spray actuator with dust cover, a bottle of nasal spray solution, and
a package insert. One
bottle will deliver 4 doses (NDC 49884-270-86).
PHARMACIST ASSEMBLY INSTRUCTIONS FOR NASCOBAL NASAL SPRAY
The pharmacist should assemble the Nascobal Nasal Spray unit prior to dispensing to the
patient, according to the
following instructions:
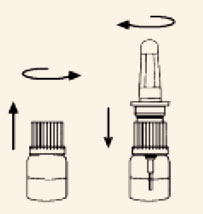
- Open the carton and remove the spray actuator and spray solution bottle.
- Assemble Nascobal Nasal Spray by first unscrewing the white cap from the spray
solution bottle and screwing
the actuator unit tightly onto the bottle. Make sure the clear dust cover is on the
pump unit.
- Return the Nascobal Nasal Spray bottle to the carton for dispensing to the
patient.
Storage Conditions
Protect from light. Keep covered in carton until ready to use. Store upright at
controlled room temperature 15°C
to 30°C (59°F to 86°F). Protect from freezing.
To report suspected adverse reactions, contact Par Pharmaceutical Companies, Inc. at
1-800-828-9393.





